Celiac Disease and Autoimmune Thyroid Diseases: The Interlink and Overlap
By: Samhitha Maddu
Antigens are macromolecules capable of stimulating an immune response in the human body by secreting immunoglobulins, commonly known as antibodies. The antigens are usually foreign and originate outside the body, and examples include viruses and microorganisms. In certain individuals, the immune system produces antibodies (proteins that defend against harmful substances called antigens) that attack healthy parts of the body (organs, intestines, bones, etc.). This leads to the development of what is known as an “autoimmune disease.” Autoimmune diseases are characterized by the “failure to distinguish self from non-self” (Wang et al., 2015). Some examples of autoimmune diseases include: rheumatoid arthritis, autoimmune thyroiditis, Hashimoto’s thyroiditis, Type 1 diabetes, Celiac disease, Crohn’s disease, etc. Recognizing the overlap between two of the most prominent autoimmune diseases–celiac disease and autoimmune thyroid diseases–may help physicians curate the intervention and treatment plan.
Celiac disease (CD) is an autoimmune, chronic digestion disorder, that affects the small bowel (Fasano, A., & Catassi, C., 2012). Occurring in genetically predisposed individuals, it causes the immune system to attack and atrophy the villous lining along the small intestine when the affected individual consumes gliadin (a component of gluten found in wheat, barley, and rye) (Levy et al., 2014). CD commonly presents with symptoms such as diarrhea, bloating, and other gastrointestinal issues (Therrien et al., 2022). It is most commonly diagnosed through serological tests and a biopsy of the small intestine. However, the only known treatment for CD is upholding a strict, lifelong, gluten-free diet. While the immediate effects of CD are not grave, the long-term effects can be fatal, often resulting in the development of other autoimmune diseases, malnutrition, osteoporosis, and increased susceptibility to certain types of cancer.
Autoimmune thyroiditis diseases (AITD), are inflammatory, chronic conditions, where the immune system mistakenly attacks the thyroid gland. The thyroid gland is an endocrinal gland that produces hormones such as triiodothyronine (T3) and tetraiodothyronine (T4). These hormones help regulate weight, metabolism, skin, hair, and nail growth, and play important roles in the endocrine system (The Endocrine Society, 2022). The two main presentations of AITD are Graves’ disease (which results in an overactive thyroid) and Hashimoto’s thyroiditis (which results in an underactive thyroid) (Antonelli et al., 2015).
The association between autoimmune thyroid diseases (AITD) and celiac disease (CD) has been extensively documented; individuals affected with one autoimmune condition show higher susceptibility to develop additional autoimmune disorders. The co-occurence of AITD and CD represents the most frequently observed overlaps in research. Even with variation in data across populations and study methodologies, a considerable amount of evidence is available to support the intersection between the two diseases. The association can be related to the shared genetic susceptibilities, in particular to HLA systems, such as the HLA-B8, HLA-D3, HLA-DQ2 and HLA-DQ8 (Lundin & Wijmenga, 2015). These genetic markers have been found in patients with either or both conditions, indicating a common immunogenetic pathway.
Researchers found a phenomenon, referred to as genetic overlap where different diseases share specific alleles or genes or sometimes loci (Sategna-Guidetti, C., et al., 2001) Sharing these common genetic factors can imply similar biological pathways, or immune mechanisms, or autoimmune disorders.
Figure 1. AITD susceptible genes (Zhang et al., 2024)
Susceptibility genes refer to the genes that increase an individuals’ likelihood of developing a particular disease or disorder, however they do not directly cause the disease on their own. The research on the development of susceptibility genes confirmed the role of genetic overlap and inheritance in AITD. Specific AITD susceptible genes are divided into two groups, such as thyroid specific (Tg, TSHR) and immune modulating (FOXP3, CD25,CD40, CTLA-4, HLA), with HLA-DR3 carrying the high risk susceptibility. CD40, CTLA-4, and HLA genes are critical for activating the T-lymphocytes and antigens, whereas FOXP3 and CD25 play crucial roles in formation of peripheral tolerance (Lee, et al., 2015).
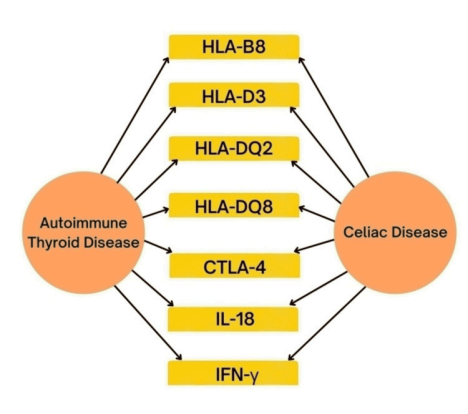
Figure 2. Shared genes between celiac disease and autoimmune thyroid disease (Tejaswini et al., “2022”)
The genes mentioned in fig. 2 are part of the major histocompatibility complex (MHC), which plays a key role in immune system function by presenting antigens to immune cells and allowing the body to recognize and respond to pathogens and/or infections. MHC is a group of genes that code for MHC proteins, also known as Human Leukocyte Antigen (HLA). When Lorini et al. (1986) compared the antigen in two patients with Graves' and CD, HLA-B8 and HLA-DR3 antigens were noted in both patients. This led them to conclude that the presence of these antigens was connected with the increased prevalence of CD and autoimmune endocrine diseases. Furthermore, these antigens also implied the patients’ genetic predisposition for these autoimmune diseases.
Acknowledging this connection will increase awareness among both healthcare providers and patients, leading to early implementation of screening protocols and potential diagnosis (of CD or AITD). Protocols such as serological testing and the genetic profiling of known susceptibility genes will help detection and disease management in those genetically predisposed individuals. Currently, serological testing is already being used for the diagnosis of CD, to measure IgG and IgA antibodies. The consistent screening of CD in patients with AITD (or vice versa), would also help with the early detection of other autoimmune disorders. However, current genetic risk profiling and serologicals tests are not optimal as diagnostic tools (Lundin et al., 2015). Genetic profiling lacks precision of disease development and serological testing falls short due to the high amount of false-negative results. Therefore it is imperative that further research is conducted for the optimization of cost-effective diagnosis and the intervention of autoimmune diseases.
References:
Wang, L., Wang, F. S., & Gershwin, M. E. (2015). Human autoimmune diseases: a comprehensive update. Journal of internal medicine, 278(4), 369–395.
Fasano, A., & Catassi, C. (2012). Celiac disease. New England Journal of Medicine, 367(25), 2419-2426.
Levy, J., Bernstein, L., & Silber, N. (2014). Celiac disease: an immune dysregulation syndrome. Current problems in pediatric and adolescent health care, 44(11), 324–327. https://doi.org/10.1016/j.cppeds.2014.10.002
Therrien, A., Kelly, C. P., & Silvester, J. A. (2020). Celiac Disease: Extraintestinal Manifestations and Associated Conditions. Journal of clinical gastroenterology, 54(1), 8–21.
The Endocrine Society (2022). Thyroid and Parathyroid Hormones. The Endocrine Society: https://www.endocrine.org/patient-engagement/endocrine-library/hormones-and-endocrine-functi on/thyroid-and-parathyroid-hormones
Antonelli, A., Ferrari, S. M., Corrado, A., Di Domenicantonio, A., & Fallahi, P. (2015). Autoimmune thyroid disorders. Autoimmunity reviews, 14(2), 174–180.
Sategna-Guidetti, C., et al. (2001). Prevalence of thyroid disorders in untreated adult celiac disease patients and effect of gluten withdrawal: An Italian multicenter study. American Journal of Gastroenterology, 96(3), 751–757.
Zhang, W., Ding, R., Hu, Y., Wei, W., Tian, D., Qin, N., Yu, H., & Wang, X. (2024). Unraveling susceptibility genes: A contemporary overview of autoimmune thyroid diseases. International immunopharmacology, 136, 112313. https://doi.org/10.1016/j.intimp.2024.112313
Lee, H. J., Li, C. W., Hammerstad, S. S., Stefan, M., & Tomer, Y. (2015). Immunogenetics of autoimmune thyroid diseases: A comprehensive review. Journal of autoimmunity, 64, 82–90.
Tejaswini, Ashok, T., Patni, N., Fatima, M., Lamis, A., & Siddiqui, S. W. (2022). Celiac Disease and Autoimmune Thyroid Disease: The Two Peas in a Pod. Cureus, 14(6), e26243.
Lorini, R., Larizza, D., Scotta, M. S., & Severi, F. (1986). HLA in Graves' disease coexistent with coeliac disease. European journal of pediatrics, 145(3), 241.
Lundin, K. E., & Wijmenga, C. (2015). Coeliac disease and autoimmune disease-genetic overlap
and screening. Nature reviews. Gastroenterology & hepatology, 12(9), 507–515.